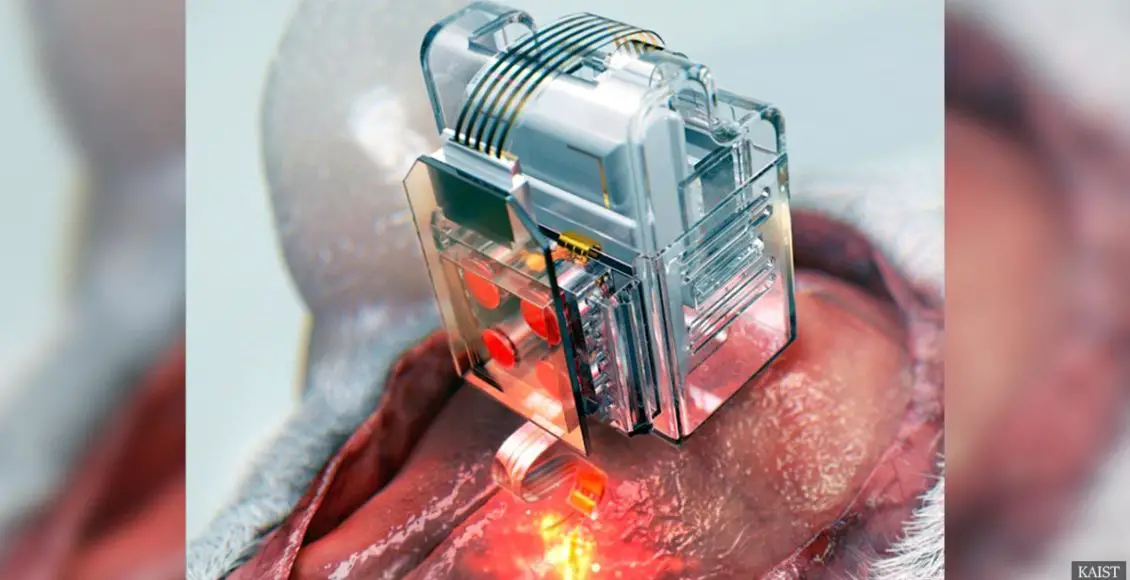
A group of scientists in the U.S. and Korea have created a device that can control neural circuits by using a miniature implant controlled by a smartphone.
Scientists publishing in Nature Biomedical Engineering, believe the device can contribute to speeding up efforts to uncover brain diseases such as Alzheimer’s, Parkinson’s, depression, addiction, and pain.
The device can target specific neurons of interest by using light and drug for prolonged periods. It uses replaceable, Lego-like drug cartridges and powerful Bluetooth low-energy.
“The wireless neural device enables chronic chemical and optical neuromodulation that has never been achieved before,” says lead author Raza Qazi, a scientist with the Korea Advanced Institute of Science and Technology (KAIST) and University of Colorado Boulder.
Mr. Qazi states that conventional methods used by neuroscientists are completely overshadowed by the new technology. Previously, the process involved optical fibers and rigid metal tubes to deliver light and drugs. And aside from limiting the movement of the subject due to the physical connections with bulky equipment, their relatively stiff structure caused damage to soft brain tissue over time, hence making them unsuitable for long-term implantation. Although some attempts have been made to partly mitigate unfavorable tissue, the previous solutions were limited by their inability to deliver drugs for extended periods of time as well as due to their complex and bulky control setups.
In order to make chronic wireless drug delivery a reality, researchers needed to solve the urgent challenge of exhaustion and evaporation of drugs.
Scientists from the Korea Advanced Institute of Science and Technology and the University of Washington in Seattle teamed up to invent a neural device with a replaceable drug cartridge, which could then allow neuroscientists to study those same brain circuits for months without having to worry about running out of drugs.
The new drug cartridges were assembled into a brian implant for mice with an extra-thin and soft probe, which was made of tiny LEDs and microfluid channels for light delivery and unlimited drug doses.
Controlled with a state of the art user interface on a smartphone, scientists can effortlessly trigger any combination or precise sequencing of light and drug deliveries in any target animal with an implant without having to be inside the laboratory.
By using wireless neural devices, researchers could also swiftly set up fully automated animal studies where the behavior of one animal could affect behavior in other animals -positively or negatively – by conditional triggering of light and/or drug delivery.
“This revolutionary device is the fruit of advanced electronics design and powerful micro and nanoscale engineering,” stated Jae-Woong Jeong, a professor of electrical engineering at KAIST. “We are interested in further developing this technology to make a brain implant for clinical applications.”
“It allows us to better dissect the neural circuit basis of behaviour, and how specific neuromodulators in the brain tune behaviour in various ways,” he said. “We are also eager to use the device for complex pharmacological studies, which could help us develop new therapeutics for pain, addiction, and emotional disorders.” said Michael Bruchas a professor of anesthesiology and pain medicine and pharmacology at the University of Washington School of Medicine
The researchers at the KAIST Jeong group create soft electronics for implantable and wearable devices, and the Bruchas lab neuroscientists at the University of Washington examine brain circuits that control addiction, stress, depression, pain, as well as other neuropsychiatric disorders.
This international collaboration among neuroscientists and engineers over a three year period which included numerous design iterations led to the successful creation of this powerful brain implant in freely moving mice, which scientists believe can truly speed up the uncovering of the brain and its diseases.