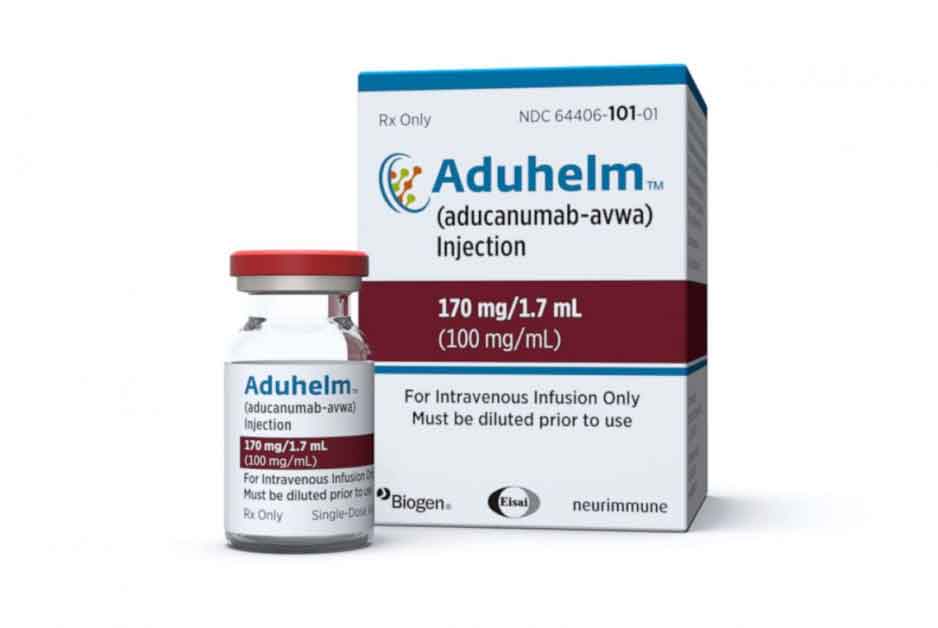
The U.S. Food and Drug Administration approved the use of Aduhelm – a new drug for treating early Alzheimer’s disease.
- The FDA approved a drug for Alzheimer’s treatment for the first time in history.
- Aduhelm is said to be treating the disease in its early stages.
- While some experts question the drug’s potential, others believe it provides a new hope for those suffering from Alzheimer’s.
The FDA approved a drug for treating Alzheimer’s for the first time ever. The drug in question is called Aduhelm(aducanumab) and is said to be treating the disease in its early stages, ABC News reports.
Dr. Babak Tousi, who led Cleveland Clinic’s study site for the multicenter clinical trials, said:
“This is the first time we have any new approved treatment for Alzheimer’s disease over the past two decades.”
The new drug is made by the biotechnology company Biogen. Prior to its approval, there has been controversy regarding its development, with some specialists arguing that it is unlikely to outweigh the risk of severe side effects.
However, the Food and Drug Administration decided to recognize the medicament but reserved their right to rescind the approval in the future.
In an official statement, they acknowledged Aduhelm has “differing perspectives,” adding that the data supporting its approval was “highly complex” and left “residual uncertainties.”
Patrizia Cavazzoni, M.D., director of the FDA’s Center for Drug Evaluation and Research, explained:
“Alzheimer’s disease is a devastating illness that can have a profound impact on the lives of people diagnosed with the disease as well as their loved ones. Currently available therapies only treat symptoms of the disease; this treatment option is the first therapy to target and affect the underlying disease process of Alzheimer’s. As we have learned from the fight against cancer, the accelerated approval pathway can bring therapies to patients faster while spurring more research and innovation.”
Following the approval, the FDA now wants Biogen to conduct a new trial to determine Aduhelm’s benefits. What is more, the administration reserves the right to withdraw the approval in the future. They wrote:
“At the end of the day, we followed our usual course of action when making regulatory decisions in situations where the data are not straightforward.”
“The trials give us hope.” – The novel drug is promising a new treatment option for millions of patients suffering from Alzheimer’s.
Dr. Jennifer Ashston, ABC Chief Medical Correspondent, commented:
“This is the first time the FDA has conditionally approved a drug for the early treatment of Alzheimer’s in 20 years. This is something that is potentially encouraging news for the 1 in 9 Americans over the age of 65 who are facing a diagnosis of Alzheimer’s.”
Aduhelm is developed to function as an antibody targeted against plaques that are found in brains affected by Alzheimer’s disease. The FDA describes the drug as an “amyloid beta-directed antibody indicated for the treatment of Alzheimer’s disease.”
Ron DeChant, a retired pharmacist and patient with Alzheimer’s disease, noted:
“The trials give us hope. I entered the study in 2016 knowing the risks involved but was encouraged that we were doing all we could to learn about Alzheimer’s disease.”
According to experts, the medicament could help Alzheimer’s patients in the early stages of the disease.
Dr. Tousi remarks:
“Millions of people will be eligible for treatment. Aducanumab is a medication given by infusion once a month. This means we need a community response to figure out how to give the medication and monitor for safety.”
Professionals involved in the trials admit the implementation phase will take time. Dr. Richard Isaacson, director of the Alzheimer’s Prevention Clinic at Weill Cornell Medicine and New York-Presbyterian, said:
“People with early-stage Alzheimer’s, their family members, and physicians will need to have conversations about the potential benefits, risks, and costs. A decision on whether or not to treat will be generally made on a case-by-case basis – since different people with different genetics, for example, may be at higher risk of side effects and need to be tracked more closely using periodic MRI scans.”
Dr. Joel Salinas, a neurologist at New York University, who is not connected to the trials, added:
“Some of the potential side effects are brain swelling and some tiny bleeding in the brain. This is still light-years from where we were. I think studies should continue now that the drug is approved. For people who get the treatment, even if it just means having a couple years of stability, that’s still incredibly meaningful for them.”
The neurologist claims that the FDA approval of Aduhelm could inspire other companies to work in that direction.
DeChant, the patient who was part of the ENGAGE trial, reveals:
“I am not the same person that I was six years ago after being diagnosed with Alzheimer’s disease. I feel like I am in a constant fog every day. I find it very frustrating just trying to perform daily activities on my own. I never needed to rely on others and I am very sad that now I need assistance with many things.”
The retired pharmacist added:
“We need to continue to find answers to treat this disease. I am willing to do anything it takes to find the cure.”


